Cortisol is a vital hormone with several important functions in the human body. Apart from its widely known function of being the body’s stress hormone, it also contributes to glucose metabolism, regulation of blood pressure, and suppression of inflammation 1. Additionally, cortisol plays a key role in regulating the circadian rhythm. In the morning, the body experiences a surge of cortisol known as the cortisol awakening response, which helps to transition the body from sleep to wakefulness. As the day progresses, cortisol levels gradually decrease, allowing melatonin to rise and induce sleep 1,2. Therefore, the disruption of this rhythm can have significant implications on sleep and by extension recovery and repair.
Given the central role this hormone plays in physiology, cortisol has been the focus of much investigation. In terms of stress, the bell-shaped cortisol curve is a well-known phenomenon in which small bursts of cortisol increase cognitive and physical output, whereas long-term elevation of cortisol is associated with depressed metabolic output and mental health problems 3. Work-related changes in cortisol levels have also been reported. For example, night shift workers show a decreased cortisol awakening response and an overall increased total cortisol secretion throughout the day 4. Furthermore, chronic stress 3 and people suffering from insomnia 5 also exhibit increased cortisol secretion.
The Ultrahuman Stress Rhythm score allows users to track their stress levels throughout the day using heart rate as the main data stream. The main aim is to help users regulate their stress synchronised with their circadian rhythm and preferably by minimising stress in the evening. However it is relevant to examine whether the actual biomarker of stress- cortisol- has any relationship with sleep quality among the Ring AIR users. Hence the motivation for the present analysis was to test whether evening to night-time cortisol levels predict the sleep quality of the following night.
The study involved five volunteers from the Ultrahuman team (excluding data analytics to account for bias). The age range of participants spanned 25-42 years and was a mix of males (two) and females (three). The participants wore the Ultrahuman Ring AIR for a period of five days and collected salivary cortisol at four time points throughout the day: Morning between 7-9 a.m., Midday: between 11 a.m. - 1 p.m., Evening between 4-5 p.m., and Night between 8-10 p.m. Salivary cortisol was collected using the Potential Health Development-Acuity Lab Cortisol Test Kit (more information here). Cortisol levels were evaluated using LC-MS in a daily batch-analysis mode.
Ring AIR-derived data on heart rate, heart rate variability (HRV), and sleep score were gathered for all participants across the five days of testing. Participants also had a protocol deviation log to report collection incidents or sleep disruption which either affected data acquisition or analysis.
Due to work-related travel, mid-day time points were missed for two days for three participants. Volunteers did not have any changes in working hours, diet, or supplements/medicines taken during the study period. Stimulant intake was also reported to be identical for all participants in the study period versus earlier. This provided a data set of N=93 observations of yoked salivary cortisol with corresponding sleep session metrics.
Statistical analysis was using in-house developed Python code, and initial regression analyses using Pearson’s correlation coefficients were undertaken.
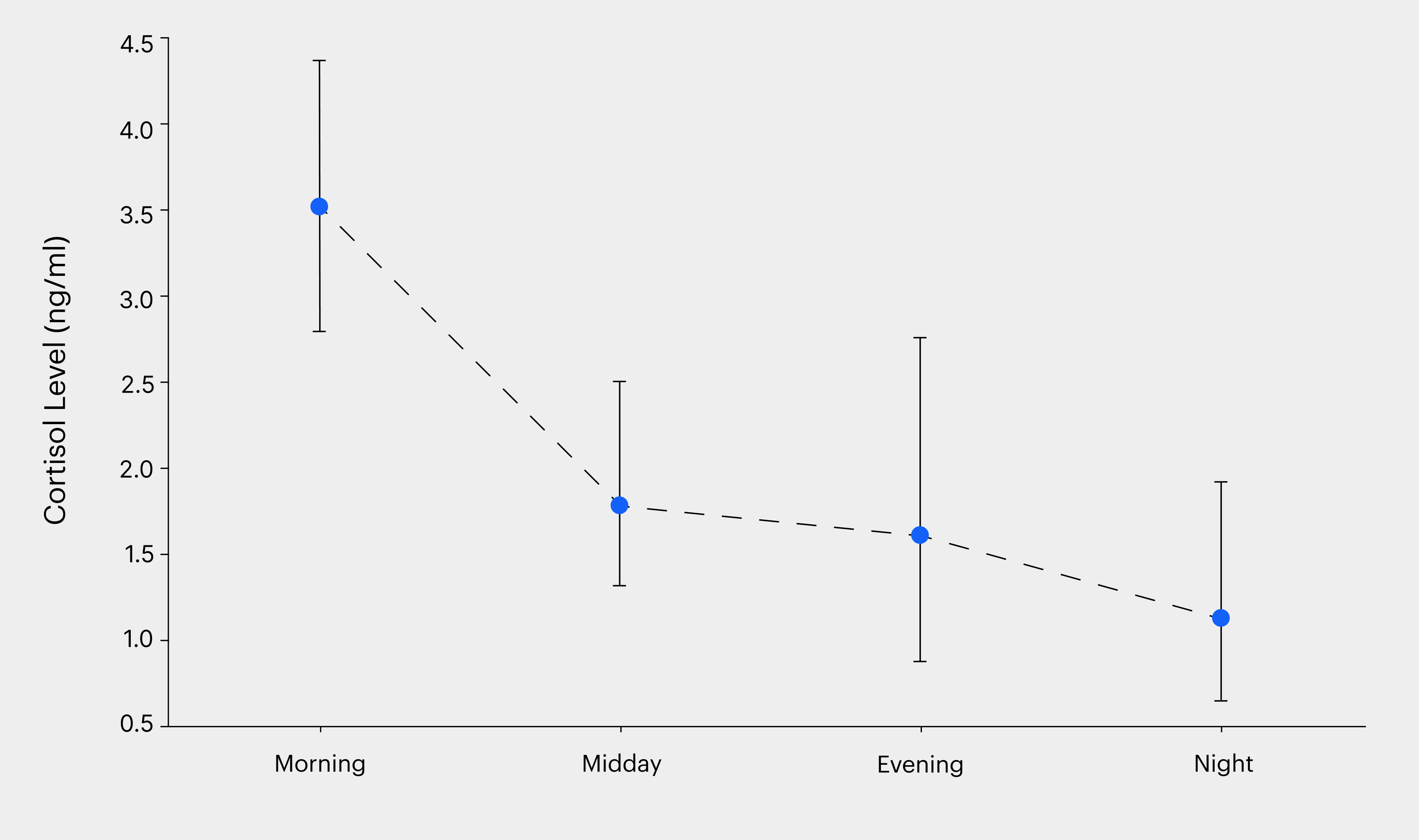
Diurnal cortisol levels followed previously established rhythms
While cortisol is expected to peak in the morning and then follow a steady decrease over the day, our test cohort may have lifestyles that may impact this rhythm. Hence it became important to test if this trend was maintained. To assess the daily salivary cortisol curve, we mapped levels at the four pre-determined intervals mentioned above. We found agreement with the known 24-hour rhythmicity of cortisol concentrations as shown in Figure 1. Our results showed higher cortisol levels in the morning (3.428 ± 1.977 ng/ml), which gradually decreased through the afternoon (1.79 ± 1.55 ng/ml) and evening (1.746 ± 3.151 ng/ml), reaching their lowest point at night (0.725 ± 0.429 ng/ml). We found no dependence on gender or age for these curves as well, indicating the universal nature of this rhythm.
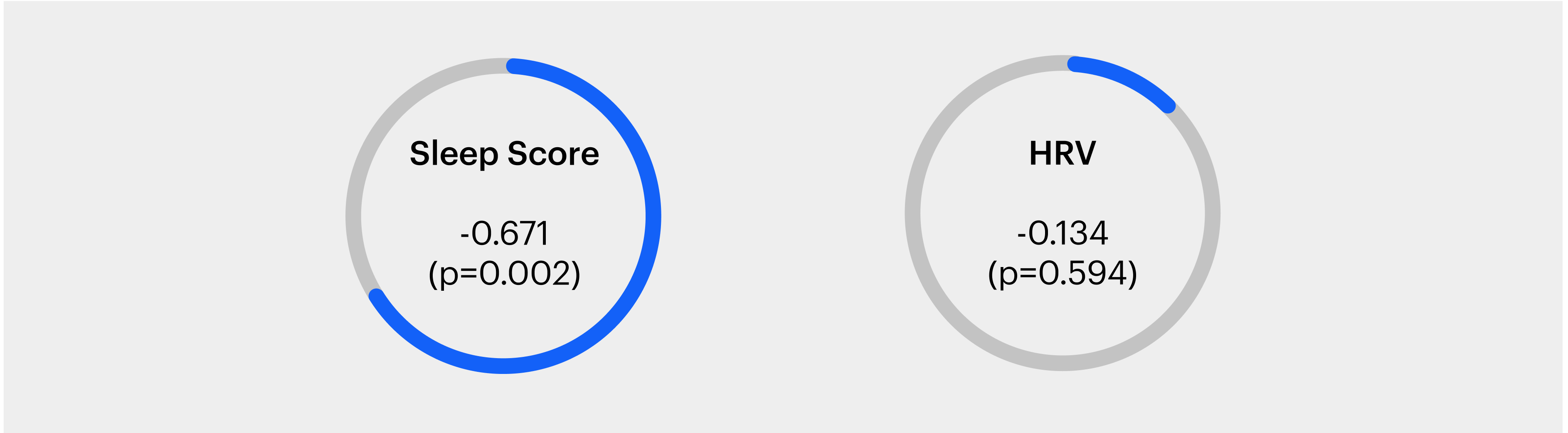
Levels of Evening Cortisol have a strong negative correlation with Sleep Score
Next, we examined the correlation between nighttime cortisol levels and the Sleep Score as well as average HRV for the sleep session (Figure 2). For this analysis, we compared the last cortisol values of the day (typically between 8-10 pm) and the app-derived Sleep Score for the following session of sleep. We observed that nighttime cortisol levels were significantly negatively correlated with sleep scores. Further, we tested a primary metric like HRV to test the strength of association with cortisol therein. With HRV we saw a weak correlation, however, this was not significant.
The production, metabolism, and secretion of cortisol are tightly regulated and usually mirror the stress load in an individual 1. Therefore, elevated nighttime levels of cortisol in healthy individuals often result from chronic stress 4, circadian misalignment (such as jet lag, and shift working) 3, and even prolonged exposure to light at night 6. However, cortisol being a rapidly metabolised biochemical entity, does not lend itself to be measured on a daily basis to monitor one’s stress levels. Instead, its influence on the cardiovascular system, via the regulation of heart rate and breathing 10, are measured using proxies like photoplethysmography (PPG) to report the stress scores. However, it is important to note that cardiovascular output as measured by PPG signals can be subject to several other internal biologic processes. Hence it becomes important to test the correlation of cortisol levels with the physiologic readout being studied.
With this motivation, we assessed the cortisol levels in volunteers from the Ultrahuman team while they engaged in usual work-life activities. We found a near-typical graph of peak cortisol in the morning with a decay through the day. While peak levels were relatively lower than reference standards indicated in the test results, there is sufficient variability in reported peak cortisol values across studies for the observed values to be considered within permissible values 11,12.
Our data provided reinforcing support for the association of elevated night-time cortisol levels with adverse effects on sleep quality. Sleep score is an aggregate output of a variety of metrics including sleep duration, times awake within a sleep period, heart rate variability, and consistency in sleeping habits. Since cortisol’s hormonal effects span cardiovascular, gastrointestinal, and neuronal centers, it is not unexpected that we obtained a stronger correlation with overall sleep score rather than a single metric of HRV. Multiple other studies have similarly indicated that heightened nighttime cortisol levels are associated with reduced REM (rapid eye movement) sleep and increased periods of wakefulness during sleep 2,7. It is equally plausible that the study sample size was not large enough to parse significant associations with HRV. In a previous retrospective analysis, we found evening heart rate based Stress Rhythm Scores were elevated for Indian Ring AIR users with evening activity, indicating a dependence on a larger sample size for this relational effect. Since this was an observational pilot study, it is our intention to follow up on the results obtained with a larger cohort.
Potential limitations of the study include the relatively small sample size and the lack of any exclusion factors employed to select the cohort, given the voluntary nature of this exercise. In participants, there may be present pre-existing conditions, lifestyle habits, chronic stress levels that can influence the outcomes obtained.
This pilot study provides preliminary evidence for the negative impact of elevated nighttime cortisol levels on sleep quality among Ultrahuman Ring AIR users. The findings underscore the importance of managing evening stress and developing effective stress management strategies for individuals whose work-life commitments expose them to regular stress closer to bedtime.
Reach out to support@ultrahuman.com for commercial queries and science@ultrahuman.com for scientific queries.
1. Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, Lightman S, Vgontzas A, Van Cauter E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr Rev. 2017 Feb 1;38(1):3-45. doi: 10.1210/er.2015-1080. PMID: 27749086; PMCID: PMC5563520.
2. Nicolaides, N.C. (2020) Hpa Axis and sleep, Endotext [Internet]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK279071/ (Accessed: 10 June 2024).
3. Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007
4. Li J, Bidlingmaier M, Petru R, Pedrosa Gil F, Loerbroks A, Angerer P. Impact of shift work on the diurnal cortisol rhythm: a one-year longitudinal study in junior physicians. J Occup Med Toxicol. 2018 Aug 14;13:23. doi: 10.1186/s12995-018-0204-y. PMID: 30123312; PMCID: PMC6090626.
5. Jan;133(1):25-45. doi: 10.1037/0033-2909.133.1.25. PMID: 17201569.
6. Xia L, Chen GH, Li ZH, Jiang S, Shen J. Alterations in hypothalamus-pituitary-adrenal/thyroid axes and gonadotropin-releasing hormone in the patients with primary insomnia: a clinical research. PLoS One. 2013 Aug 9;8(8):e71065. doi: 10.1371/journal.pone.0071065. PMID: 23951080; PMCID: PMC3739817.
7. Rahman SA, Wright KP Jr, Lockley SW, Czeisler CA, Gronfier C. Characterizing the temporal Dynamics of Melatonin and Cortisol Changes in Response to Nocturnal Light Exposure. Sci Rep. 2019 Dec 23;9(1):19720. doi: 10.1038/s41598-019-54806-7. PMID: 31873098; PMCID: PMC6928018.
8. Vgontzas AN, Zoumakis M, Bixler EO, Lin HM, Prolo P, Vela-Bueno A, Kales A, Chrousos GP. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003 May;88(5):2087-95. doi: 10.1210/jc.2002-021176. PMID: 12727959.
9. Chalmers T, Hickey BA, Newton P, Lin CT, Sibbritt D, McLachlan CS, Clifton-Bligh R, Morley JW, Lal S. Associations between Sleep Quality and Heart Rate Variability: Implications for a Biological Model of Stress Detection Using Wearable Technology. Int J Environ Res Public Health. 2022 May 9;19(9):5770. doi: 10.3390/ijerph19095770. PMID: 35565165; PMCID: PMC9103972.
10. Schulz A, Richter S, Ferreira de Sá DS, Vögele C, Schächinger H. Cortisol rapidly increases baroreflex sensitivity of heart rate control, but does not affect cardiac modulation of startle. Physiol Behav. 2020 Mar 1;215:112792. doi: 10.1016/j.physbeh.2019.112792. Epub 2019 Dec 21. PMID: 31870942.
11. Almeida DM, Piazza JR, Stawski RS. Interindividual differences and intraindividual variability in the cortisol awakening response: an examination of age and gender. Psychol Aging. 2009 Dec;24(4):819-27. doi: 10.1037/a0017910. PMID: 20025398; PMCID: PMC2810505.
12. Henckens MJ, Klumpers F, Everaerd D, Kooijman SC, van Wingen GA, Fernández G. Interindividual differences in stress sensitivity: basal and stress-induced cortisol levels differentially predict neural vigilance processing under stress. Soc Cogn Affect Neurosci. 2016 Apr;11(4):663-73. doi: 10.1093/scan/nsv149. Epub 2015 Dec 14. PMID: 26668010; PMCID: PMC4814795.